Green fishing of cephalosporin antibiotics
 Cephalosporins are life-saving antibiotics that are on the market for
approximately 25 years. Their markets will probably remain substantial for
the next two decades. Accordingly, there is both the opportunity and the need
for innovation of the processes by which these antibiotics are being
manufactured.
An important driving force behind these innovations is
reduction of the amount of chemical waste.This can be achieved by replacing
the chemical processes by "green" processes.
Cephalosporins are life-saving antibiotics that are on the market for
approximately 25 years. Their markets will probably remain substantial for
the next two decades. Accordingly, there is both the opportunity and the need
for innovation of the processes by which these antibiotics are being
manufactured.
An important driving force behind these innovations is
reduction of the amount of chemical waste.This can be achieved by replacing
the chemical processes by "green" processes.
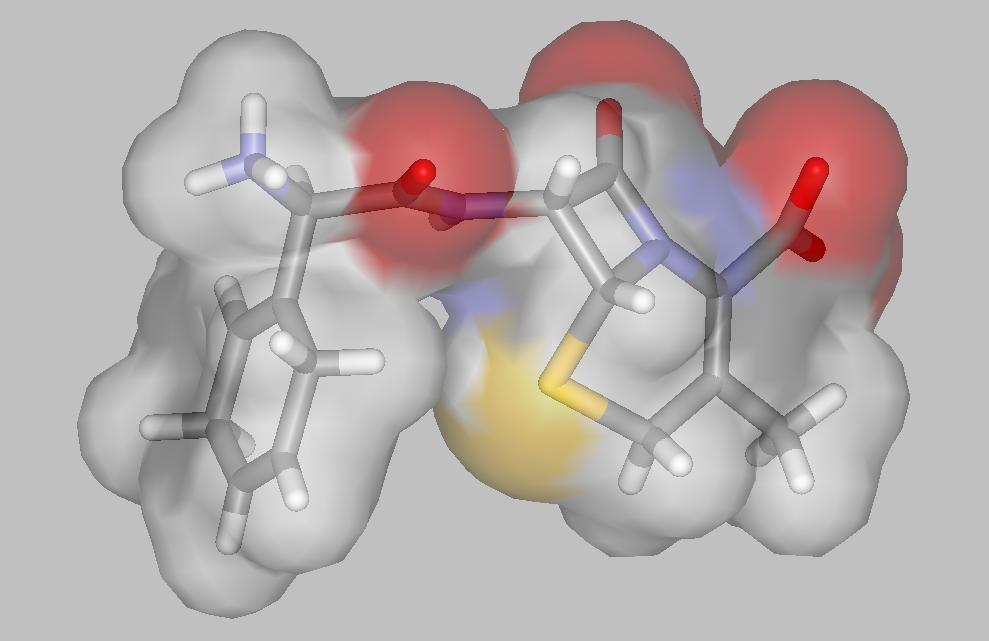
Cephalosporins are semi-synthetic
antibiotics. They consist of a beta-lactam nucleus and a D-amino acid side chain.
The final step in the preparation of these cephalosporins comprises the
coupling of the beta-lactam nucleus and the D-amino acid side chain. The
conventional method to achieve this coupling requires stoichiometric
chemistry resulting in the formation of large amounts of chemical waste.
A contemporary method employs biocatalysis for the coupling of the nucleus
and side chain (in water). An important drawback of the enzymatic coupling is
secondary hydrolysis of the cephalosporin product by the enzyme. In addition,
isolation of the product from the aqueous reaction mixture is hampered because
product concentrations obtained by the enzymatic coupling are relatively
low.
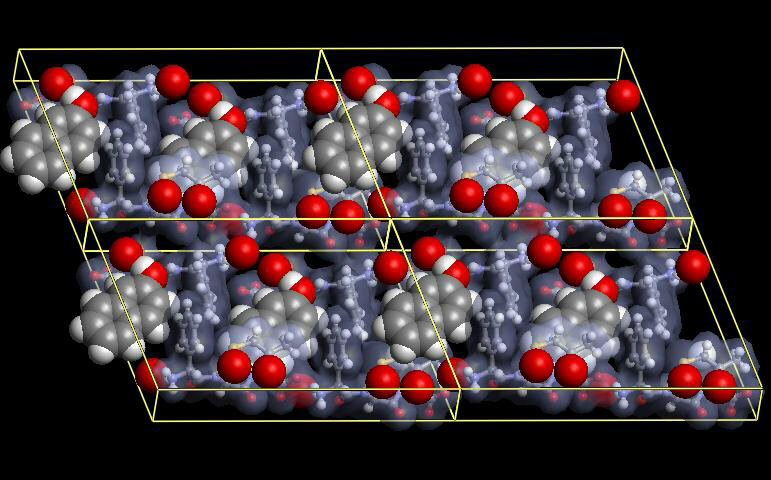
Selective complexation of the cephalosporins provides an efficient method
for the isolation of these antibiotics from aqueous solutions. Moreover, the
problem of secondary hydrolysis is strongly reduced by instantaneous withdrawal
of the product.
Beta-naphthol was already known as a reasonable complexing agent for four
cephalosporins. However, it is rather toxic and hence is not particularly
suitable for application in a "green" enzymatic process.
The antibiotics research in Nijmegen, a collaboration between Organic
Chemistry, Chemical Crystallography and DSM, was aimed at acquiring
fundamental knowledge about the selective complexation process and at a
significant improvement of the complexation process with respect to both the
efficiency and the toxicity of the complexing agents.
This is an example of Crystal Engineering research.
See also research in Chemical Crystallography.
 |
 |
| An antibiotic molecule | X-raying a crystal |
 |
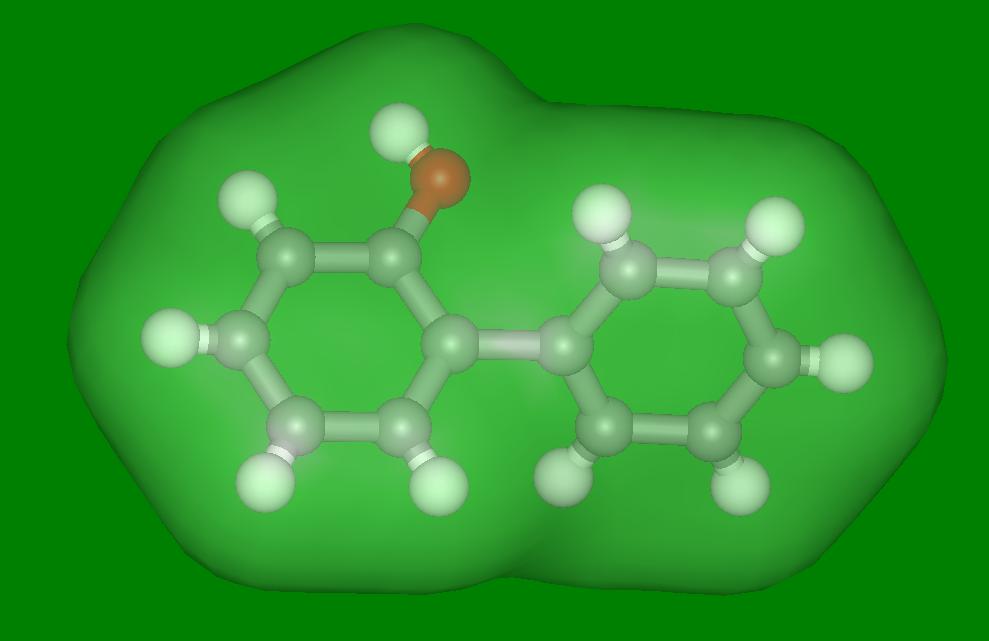 |
| Cefradine/beta-naphthol/water complex | A new green complexant |
References
- Complexen van Beta-lactam antibiotica.
G.J. Kemperman, R. de Gelder, P.C. Raemakers-Franken.
NL1007827, 1997.
- Complexes of Beta-lactam antibiotics and 1-naftol.
G.J. Kemperman, R. de Gelder, P.C. Raemakers-Franken.
NL1007828, 1997; WO99/31109.
-
Clathrate Type Complexation of Cephalosporins with Beta-naphtol.
G.J. Kemperman, R. de Gelder, F.J. Dommerholt, P.C. Raemakers-Franken, A.J.H. Klunder, and B. Zwanenburg.
Chemistry, a European Journal 5 (1999), 2163-2168.
-
Molecular Challenges in Modern Chemometrics.
R. Wehrens, R. de Gelder, G.J. Kemperman, B. Zwanenburg, and L.M.C. Buydens.
Analytica Chimica Acta (1999), 400, 413-424.
- Selective Complexation of Cephalosporin Antibiotics.
R. de Gelder, G.J. Kemperman, F.J. Dommerholt, A.J.H. Klunder, and B. Zwanenburg.
IUCr XVIII Congress and General Assembly, Collected Abstracts, C.C. Wilson, K.
Shankland and T. Csoka, Eds., (1999), 407.
-
Induced Fit Phenomena in Clathrate Structures of Cephalosporins.
G.J. Kemperman, R. de Gelder, F.J. Dommerholt, P.C. Raemakers-Franken, A.J.H. Klunder, and B. Zwanenburg.
Journal of the Chemical Society-Perkin Transactions 2 (2000), 7, 1425-1429.
- Crystallographic and Thermodynamic Aspects of Cephalosporin Complexation .
R. de Gelder, G.J. Kemperman, F.J. Dommerholt, A.J.H. Klunder, and B. Zwanenburg.
19th European Crystallographic Meeting, Nancy, France, 25th - 31st August 2000, Abstracts book, 313.
-
Efficiency of Cephalosporin Complexation with
Aromatic Compounds.
G.J. Kemperman, R. de Gelder, F.J. Dommerholt, P.C. Raemakers-Franken, A.J.H. Klunder, and B. Zwanenburg.
Journal of the Chemical Society-Perkin Transactions 2 (2001), 4, 633-638.
-
A Computational Model to Predict Clathration of Molecules with
Cephradine.
G.J. Kemperman, R. de Gelder, R. Wehrens, F.J. Dommerholt, A.J.H. Klunder, L.M.C. Buydens, and B. Zwanenburg.
Journal of the Chemical Society-Perkin Transactions 2 (2001), 6, 981-987.
- Molecular Precision in the Chemistry of Cephalosporin type Antibiotics.
B. Zwanenburg, G.J. Kemperman, J. Zhu, R. de Gelder, F.J. Dommerholt, G.T.M. Titulaer, R. Keltjens and A.J.H. Klunder.
In: Synthesis of Beta-lactam Antibiotics. Chemistry, Biocatalysis & Process
Integration. Chapter II, page 56-101. Edited by Alle Bruggink, Kluwer Academic
Publishers, 2001.
-
Complexants for the clathration mediated synthesis of the antibiotic
cephradine.
G.J. Kemperman, R. de Gelder, F.J. Dommerholt, C.G.P.H. Schroën, R. Bosma, and B. Zwanenburg.
Green Chemistry (2001), 3, 4, 189-192.
-
Cavities, Layers and Channels in the hosting framework of molecular
complexes derived from Cephradine.
G.J. Kemperman, R. de Gelder, F.J. Dommerholt, P.C. Raemakers-Franken, A.J.H. Klunder, and B. Zwanenburg.
European Journal of Organic Chemistry (2001), 3641-3650.

-
Clathrate Type Complexation of Cephalosporin Antibiotics - Function, design and application.
G.J. Kemperman.
Thesis (2001). University of Nijmegen. The Netherlands.
-
Molecular selectivity and cooperativity in clathrate-type
complexation of Cephradine.
G.J. Kemperman, R. de Gelder, F.J. Dommerholt, A.J.H. Klunder, and B. Zwanenburg.
European Journal of Organic Chemistry (2002), 345-350.
 Cephalosporins are life-saving antibiotics that are on the market for
approximately 25 years. Their markets will probably remain substantial for
the next two decades. Accordingly, there is both the opportunity and the need
for innovation of the processes by which these antibiotics are being
manufactured.
An important driving force behind these innovations is
reduction of the amount of chemical waste.This can be achieved by replacing
the chemical processes by "green" processes.
Cephalosporins are life-saving antibiotics that are on the market for
approximately 25 years. Their markets will probably remain substantial for
the next two decades. Accordingly, there is both the opportunity and the need
for innovation of the processes by which these antibiotics are being
manufactured.
An important driving force behind these innovations is
reduction of the amount of chemical waste.This can be achieved by replacing
the chemical processes by "green" processes.



